Atlantic salt marsh snake: Difference between revisions
m (Replaced content with "<div class="irlbody"> {{IRL header estuary|cat=Land Animals}} <div class="irlcontenttop"> File:Eastern_Indigo_Snake.jpg|alt=Eastern indigo snake (Drymarchon couperi)|th...") Tag: Replaced |
mNo edit summary |
||
| (29 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
<div class="irlbody"> | <div class="irlbody"> | ||
{{IRL header estuary|cat=Land Animals}} | {{IRL header estuary|cat=Land Animals}} | ||
<div class="irlcontenttop"> | <div class="irlcontenttop"> | ||
[[File: | [[File:Atlantic_salt_marsh_snake.jpg|alt=Atlantic salt marsh snake (Nerodia clarkii taeniata)|thumb|240px|'''[[Atlantic salt marsh snake]]'''<br/> ''Nerodia clarkii taeniata''<br/>Status: [[:Category:Endangered Species|Threatened]]]] | ||
<p><i><b>''Nerodia clarkii taeniata''</b></i>, commonly known as the <b>Atlantic salt marsh snake</b>, is found only in the coastal salt marshes of Brevard and Volusia, Florida. The Atlantic salt marsh snake was listed as a [[:Category:Endangered Species|threatened species]] on November 29, 1977 by the U.S. Fish and Wildlife Service.<ref name="USFWSdetermination1977"/></p> | |||
</div> | </div> | ||
==Description== | |||
<p>There are three subspecies of salt marsh snakes in Florida, the Gulf salt marsh snake (''Nerodia clarkii clarkii''), mangrove water snake (''N. c. compressicauda''), and Atlantic salt marsh snake (''N. c. taeniata'').<ref name="USFWSrecovery1993"/></p> | |||
[[File:Gulf_salt_marsh_snake.jpg|alt=Gulf salt marsh snake (Nerodia clarkii clarkii)|thumb|240px|'''Gulf salt marsh snake'''<br/> ''Nerodia clarkii clarkii'']] | |||
<p>Salt marsh snakes dorsal pattern's are formed from a basic pattern of four rows of dark blotches running from head to tail (two lateral and two dorsolateral rows) on a lighter background. In the striped forms, the blotches join to form stripes; in the banded forms, the blotches join across the back to form bands. In partially striped individuals, it is usually the anterior portion of the body that is striped, with the pattern posteriorly consisting of bands or rows of unfused blotches.</p> | |||
<p>The Gulf salt marsh snake (''Nerodia clarkii clarkii'') has a dorsal pattern that is completely striped, or nearly so, with dark brown to black stripes on a tan background. It is not unusual for the lateral stripes in this form to break down posteriorly into rows of blotches.</p> | |||
[[File:Mangrove_salt_marsh_snake_Nerodia_clarkii_compressicauda.jpg|alt=Mangrove salt marsh snake (Nerodia clarkii compressicauda)|thumb|240px|'''Mangrove salt marsh snake'''<br/> ''Nerodia clarkii compressicauda'']] | |||
<p>The mangrove water snake (''N. clarkii compressicauda'') may be uniformly orange in color, but it more often has a pattern of dark bands on a lighter background. Individuals from throughout the range of the mangrove water snake may be partially striped; in these specimens the striping is typically limited to the neck region, but occasional specimens may be more extensively striped. Coloration in the mangrove water snake is extremely variable, with the background being gray, straw, or reddish and the bands being black, brown, or red. Populations of mangrove water snakes characteristically include at least some individuals that exhibit reddish or orange pigmentation.</p> | |||
[[File:Atlantic_salt_marsh_snake_02.jpg|alt=Atlantic salt marsh snake (Nerodia clarkii taeniata)|thumb|240px|'''Atlantic salt marsh snake'''<br/> ''Nerodia clarkii taeniata'']] | |||
<p>The Atlantic salt marsh snake (''N. c. taeniata'') is a partially striped salt marsh snake that reaches a maximum length of 82 cm (32 in.), although it is typically less than 65 cm (26 in.) in length. The pattern consists of a gray to pale olive background with black to dark brown stripes anteriorly, the stripes breaking up into rows of spots posteriorly. The extent of the striping is variable, but most individuals from the coastal marshes of Volusia County are striped on at least the anterior 30 percent of the body. The venter is black with a central row of large cream to yellowish spots. As in the case of the dorsal striping, this ventral pattern is best developed anteriorly and tends to break down posteriorly. The red pigmentation characteristic of mangrove water snakes is conspicuously lacking in Atlantic salt marsh snakes from the vicinity of Edgewater, Volusia County, and northward.</p> | |||
<p>Hebrard (1979) reported coloration for 23 specimens from the southern Indian River Lagoon, near the Volusia-Brevard county line. Of these, 7 (30 percent) exhibited orange or reddish pigmentation either dorsally or ventrally. It is unclear at this time (1993) whether the reddish pigmentation reported by Hebrard should be interpreted as indicating intergradation with the mangrove water snake. The series of 25 specimens for which Hebrard provided pattern descriptions had dorsal stripes on 0 to 100 percent of the body; only 8 (32 percent) had dorsal stripes on more than 30 percent of the body, but 3 (12 percent) reportedly had dorsal stripes on 100 percent of the body. (In terms of pattern formation, the vertebral stripe is actually the lighter background color which is visible between the two dark, dorsolateral stripes.)</p> | |||
==Distribution== | |||
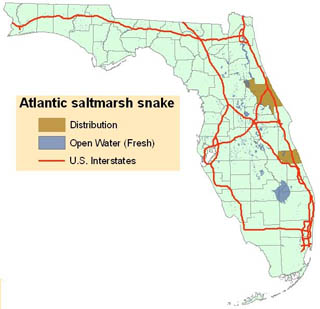
[[File:Atlantic_salt_marsh_snake_distribution_map.jpg|alt=Atlantic salt marsh snake (Nerodia clarkii taeniata) map|thumb|320px|'''Atlantic salt marsh snake distribution''']] | |||
The species to which the Atlantic salt marsh snake belongs, N. clarkii, is found in a narrow coastal strip from southern Texas, east along the Gulf coast, around the Florida peninsula, and up the east coast of Florida at least as far as Volusia County's Halifax River. | |||
Both Cope’s (1895) type series and the specimens used by Carr and Goin (1942) to resurrect N. c. taeniata came from the brackish coastal marshes of Volusia County, Florida. There is some uncertainty about the precise locality from which Cope’s specimens came, but Carr and Goin restricted the type locality to the vicinity of National Gardens, which lies near the north end of the Halifax River. Salt marsh snakes have not been documented to the north in southern Flagler County. The Carr and Goin series was collected on the barrier island at New Smyrna Beach. Recent records for populations identifiable as Atlantic salt marsh snakes are available from (1) the barrier island a short distance north of Ponce Inlet, (2) the mainland shoreline east ofthe New Smyrna Beach airport, (3) two localities on the barrier island at New Smyrna Beach (Florida Game and Fresh Water Fish Commission (FGFWFC) records), (4) an island in the Indian River east of Edgewater (G. Goode, East Volusia Co. Mosquito Control, pers. comm.) and (5) a single specimen identified as N. c. taeniata was captured just south of the Flagler County line (G. Goode pers. comm.). It is not known if a viable population exists in this area or to the north in Flagler County but if so, these Atlantic salt marsh snakes are now isolated from populations in the northern Indian River Lagoon by the Ormond Beach-Daytona metropolitan area. | |||
A problem attendant to the listing of any subspecies that is distributionally continuous and intergradient with another subspecies is the difficulty of defining the limit(s) of the listed form’s distribution in the area where it contacts the related, unlisted subspecies. To the south, the Atlantic salt marsh snake intergrades with the mangrove water snake along the central Atlantic coast of Florida. As noted above~ both the description and the resurrection of the subspecies were based on specimens from Volusia County, although Carr and Goin (1942) considered a single specimen from Indian River County also to be N. c. taeniata. They also mentioned a specimen of salt marsh snake from Melbourne, Brevard County, but did not indicate whether they considered that specimen to be N. c. taeniata. Wright and Wright (1957) considered N. c. taeniata to extend only as far south as the lower end of Mosquito Lagoon, in northern Brevard County, and Neill (1958) indicated that N. c. taeniata intergraded with the mangrove water snake on Merritt Island. In the final listing of the Atlantic salt marsh snake (FR 42:60743-60745), the Service indicated that “The Atlantic salt marsh snake is known only from coastal areas of Brevard, Volusia, and Indian River counties.” However, Hebrard and Lee (1981) examined a large series of salt marsh snakes from southern Mosquito Lagoon near the Volusia-Brevard county line and reported that they resembled Nerodia fasciata compressicauda quite closely.” Hebrard and Lee further noted that their specimens differed markedly in coloration and pattern from specimens of N. c. taeniata from further north in Volusia County. It is also worth noting that the snakes examined by Hebrard and Lee were collected in mangroves (species not indicated), whereas only about 10 miles farther north, where populations of typical Atlantic salt marsh snakes are found, the habitat consists primarily of glasswort (Salicornia spp.) flats and salt grass (Distichlis spicata)-bordered tidal creeks with only scattered black mangroves (Avicennnia germinans). The zone of intergradation appears to coincide with the increasing dominance of mangrove swamps, eventually as mangrove swamps become predominant so does N. c. compressicauda. Kochman (1992) concluded that “salt marsh snakes from farther south in Brevard and Indian River counties, although occasionally striped, appear to comprise a zone of intergradation with N. c. compressicauda.” | |||
Until a survey and taxonomic assessment have been conducted, it will not be possible to determine the southern distributional limit of the Atlantic salt marsh snake. Nonetheless, it appears that the subspecies may be restricted to the brackish, coastal marshes of Volusia County, from the Halifax River south to the northern portions of the Indian River Lagoon. | |||
==Habitat== | |||
Atlantic salt marsh snakes are restricted to brackish, tidal marshes. They most often have been found in association with saltwort flats and salt grass-bordered tidal creeks. It is not known if they occur in the adjacent black needlerush (Juncus roemerianus) habitat. Atlantic salt marsh snake use of marsh habitats may be limited by water level; with extreme fluctuations making the marsh too hydric or xeric (G. Goode pers. comm.). When inactive or pursued, they frequently retreat into one of the numerous fiddler crab (Uca pugilator) burrows that riddle the edge of the marsh and the banks of the tidal creeks (Carr and Goin 1942, Kochman 1992, P. Moler pers. obs.). | |||
==Life History/Ecology== | |||
Although the Atlantic salt marsh snake is most easily observed at night, it may be active at any time of day. Its activity is influenced by tidal cycles, which strongly influence the availability of food (Neill 1958). Although Carr and Goin (1942) indicated that all of their specimens were collected “just as the tide was beginning to overflow the flats,” Kochman (1992) indicated that it was observed most often “during low tidal stages, when it apparently feeds on small fishes that become trapped in the shallow water.” It feeds primarily on small fish, but it readily takes frogs when available. | |||
This species is ovoviviparous. Captive individuals have given birth to 3 to 9 young from August to October (Kochman 1992). Fecundity is low relative to the adjacent freshwater species, N. fasciata, which may give birth to 50 or more young. | |||
Most snakes adapted to life in salt water (families Hydrophiidae, Achrocordidae, and Homalopsidae) possess salt glands, through which they excrete excess salts (Dunson 1975). The salt marsh snakes apparently lack salt glands (Schmidt-Nielsen and Fange 1958), but they nonetheless exhibit very low dehydration rates in seawater (Pettus 1963, Dunson 1978, 1980). Salt marsh snakes are apparently able to survive in seawater through their reduced rates of cutaneous water and salt exchange and their refusal to drink seawater even when they become dehydrated. By contrast, when held in seawater, their freshwater congeners quickly become dehydrated, which prompts them to drink. This merely exacerbates their dehydration and leads to death (Pettus 1963). Salt marsh snakes readily drink fresh water when it becomes available from rain or dew (Kochman 1992). | |||
==Threats== | |||
The Atlantic salt marsh snake was listed on the basis of two primary concerns, intensive drainage and development in coastal salt marshes resulting in loss of habitat and the accompanying disruption of reproductive isolating mechanisms, leading to hybridization with the Florida banded water snake and potential swamping ofthe Atlantic salt marsh snake gene pool by the much larger Florida banded water snake gene pool. | |||
At the time of its listing, the Atlantic salt marsh snake was thought to include salt marsh snakes as far south as Indian River County (U.S. Fish Wildlife Service 1977). As suggested above, it may actually be much more restricted, occurring only in the brackish, coastal marshes of Volusia County. If so, then given its highly restricted distribution, the Atlantic salt marsh snake’s vulnerability to habitat destruction and modification is even greater than previously realized. | |||
It is well known that salt marsh snakes occasionally hybridize with the closely related freshwater species, Nerodia fasciata, especially in areas of habitat disturbance (Kochman 1977, Dunson 1979, Lawson et al. 1991). Lawson ~ ~j. (1991) demonstrated that, despite the reproductive compatibility of the two forms, there appears to be little or no genetic introgression between them in areas of undisturbed habitat. The extent of genetic introgression associated with the local breakdown of reproductive isolation between the two species has not yet been examined. | |||
Rising sea levels are not an immediate threat but in the long termmay reduce the amount of habitat available to the Atlantic salt marsh snake. As sea levels rise, salinity in the estuaries will also rise correspondingly and possibly change the vegetation ofthe marsh, eventually flooding the area and making it inhospitable for the snake. | |||
==Conservation Measures== | |||
Conservation measures have consisted of limited survey work, genetic comparison with other salt marsh snakes and southern banded water snakes, use ofthe provisions under Section 7 of the Act, Section 404 of the Clean Water Act (CWA), and the Fish and Wildlife Coordination Act (FWCA), and proposals for creation of habitat to mitigate for areas impacted by permitted dredge-and-fill activities. | |||
Sporadic surveys conducted from 1978 to 1988 by personnel of the FGFWFC and the Service confirmed the continued presence of the Atlantic salt marsh snake at several localities in Volusia County, Florida. Personnel of the East Volusia County Mosquito Control District are currently conducting surveys for Atlantic salt marsh snakes associated with mosquito control impoundments on islands in the northern portions of the Indian River Lagoon (G. Goode pers. comm.). A survey was conducted on Merritt Island National Wildlife Refuge in the late 1970’s, and a large population of salt marsh snakes was identified in the vicinity of the Volusia-Brevard county line, but this population seemed to show signs of intergradation with the mangrove water snake (Hebrard and Lee 1981). | |||
Localities in the vicinity of New Smyrna Beach were sampled by FGFWFC for genetic studies (Lawson et M. 1991). Electrophoretic analyses indicated that the salt marsh snakes are closely related to but specifically distinct from the southern banded water snake (Nerodia fasciata), and that the three subspecies of the salt marsh snake are electrophoretically indistinguishable from each other (Lawson et al. 1991). Tissues were saved for possible comparison of mitochondrial DNA variation in the salt marsh snakes, but that work has not yet been performed. | |||
The Atlantic salt marsh snake is protected as a threatened species under the Endangered Species Act of 1973, as amended (16 U.S.C. 1531 ~4seq.). The Act places an affirmative mandate on Federal agencies to carry out programs for the conservation of federally listed endangered and threatened species. Further, the Act requires all Federal agencies to ensure that their actions are not likely to jeopardize the continued existence of any federally listed endangered or threatened species. Federal agency actions that can directly or indirectly affect endangered or threatened species include any activity that is authorized, funded, or carried out by such agency. Compliance with theses standards is ensured under Section 7 of the Act because agencies must consult with the Service or National Marine Fisheries Service on actions that may affect listed species or critical habitat. | |||
In addition to Section 7 consultations, protection and conservation of salt marsh habitat is provided by CWA and FWCA. The Service and U.S. Army Corps of Engineers review proposed dredge-and-fill activities and construction projects in waters of the United States where projects may affect the Atlantic salt marsh snake or its habitat. During a 10-year period (1983-1992) a minimum of 36 various projects were permitted in Volusia County’s salt marsh habitat. These projects included dredge-and-fill, shoreline protection projects, construction of piers and marinas, mosquito ditching, and water control structures. However, only 32 acres of salt marsh were destroyed by these projects, most (29.44 acres, 18 projects) before 1988. Loss of salt marsh habitat appears to have slowed since 1988 (2.56 acres, 18 projects) indicating improved protection. If the Atlantic salt marsh snake is limited to Volusia County, any project destroying salt marsh habitat may be detrimental to the species. | |||
<div class="irlcontentbottom noprint"> | <div class="irlcontentbottom noprint"> | ||
==Video== | |||
<p>University of Central Florida - So where are all the Atlantic salt marsh snakes? We know that changes in our environment affect our ecosystem, and now biologists are wondering if manmade changes to the snake habitat in Volusia County are making them all but disappear in this segment of WUCF TV's "ONE." | |||
<youtube>https://youtube.com/watch?v=NbDd9dJm0Yg</youtube> | |||
==Documents== | ==Documents== | ||
* [https://www.fws.gov/ | * [https://www.govinfo.gov/content/pkg/FR-1977-11-29/pdf/FR-1977-11-29.pdf#page=35 Federal Register - USFWS Determination for Atlantic Salt Marsh Snakes 1977 (PDF 168pp 43MB)]. Retrieved May 23, 2022. | ||
* [https://ecos.fws.gov/docs/recovery_plan/931215.pdf USFWS Atlantic Salt Marsh Snake Recovery Plan 1993 (PDF 24pp 1.6MB)]. Retrieved May 23, 2022 | |||
* [https://www.fnai.org/PDFs/FieldGuides/Nerodia_clarkii_taeniata.pdf FNAI - Atlantic salt marsh snake (''Nerodia clarkii taeniata'') (PDF 2pp)] | |||
* [https://ecos.fws.gov/docs/tess/species_nonpublish/2908.pdf ECOS - Atlantic salt marsh snake - Nerodia clarkii taeniata - 2019 Review (PDF 19pp)]. Retrieved May 23, 2022. | |||
==Web Links== | ==Web Links== | ||
* [https://myfwc.com/wildlifehabitats/profiles/reptiles/snakes/ | * [https://www.fws.gov/species/atlantic-salt-marsh-snake-nerodia-clarkii-taeniata USFWS Atlantic salt marsh snake (''Nerodia clarkii taeniata'')] | ||
* [https://ecos.fws.gov/ecp/species/7729 ECOS Atlantic salt marsh snake] | |||
* [https://myfwc.com/wildlifehabitats/profiles/reptiles/snakes/atlantic-salt-marsh-snake/ FWC Atlantic salt marsh snake Nerodia clarkii taeniata profile] | |||
* [https://eol.org/pages/1243758 EOL Atlantic salt marsh snake (''Nerodia clarkii clarkii'')] | |||
* [https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=209380#null ITIS Nerodia clarkii taeniata] | |||
==References== | ==References== | ||
<references/> | <references> | ||
<ref name="USFWSdetermination1977">[https://www.govinfo.gov/content/pkg/FR-1977-11-29/pdf/FR-1977-11-29.pdf#page=35 Federal Register - USFWS - Determination for Atlantic Salt Marsh Snakes 1977 (PDF 168pp 43MB)]. Article source. Published 1977-11-29. Retrieved 2022-05-23.</ref> | |||
<ref name="USFWSrecovery1993">[https://ecos.fws.gov/docs/recovery_plan/931215.pdf USFWS Atlantic Salt Marsh Snake Recovery Plan 1993 (PDF 24pp 1.6MB)]. Published 1993-12-15. Retrieved 2022-05-23.</ref> | |||
</references> | |||
{{IRL footer estuary|cat=Biota}} | {{IRL footer estuary|cat=Biota}} | ||
</div> | </div> | ||
Latest revision as of 09:03, May 30, 2022
Nerodia clarkii taeniata, commonly known as the Atlantic salt marsh snake, is found only in the coastal salt marshes of Brevard and Volusia, Florida. The Atlantic salt marsh snake was listed as a threatened species on November 29, 1977 by the U.S. Fish and Wildlife Service.[1]
Description
There are three subspecies of salt marsh snakes in Florida, the Gulf salt marsh snake (Nerodia clarkii clarkii), mangrove water snake (N. c. compressicauda), and Atlantic salt marsh snake (N. c. taeniata).[2]
Salt marsh snakes dorsal pattern's are formed from a basic pattern of four rows of dark blotches running from head to tail (two lateral and two dorsolateral rows) on a lighter background. In the striped forms, the blotches join to form stripes; in the banded forms, the blotches join across the back to form bands. In partially striped individuals, it is usually the anterior portion of the body that is striped, with the pattern posteriorly consisting of bands or rows of unfused blotches.
The Gulf salt marsh snake (Nerodia clarkii clarkii) has a dorsal pattern that is completely striped, or nearly so, with dark brown to black stripes on a tan background. It is not unusual for the lateral stripes in this form to break down posteriorly into rows of blotches.
The mangrove water snake (N. clarkii compressicauda) may be uniformly orange in color, but it more often has a pattern of dark bands on a lighter background. Individuals from throughout the range of the mangrove water snake may be partially striped; in these specimens the striping is typically limited to the neck region, but occasional specimens may be more extensively striped. Coloration in the mangrove water snake is extremely variable, with the background being gray, straw, or reddish and the bands being black, brown, or red. Populations of mangrove water snakes characteristically include at least some individuals that exhibit reddish or orange pigmentation.
The Atlantic salt marsh snake (N. c. taeniata) is a partially striped salt marsh snake that reaches a maximum length of 82 cm (32 in.), although it is typically less than 65 cm (26 in.) in length. The pattern consists of a gray to pale olive background with black to dark brown stripes anteriorly, the stripes breaking up into rows of spots posteriorly. The extent of the striping is variable, but most individuals from the coastal marshes of Volusia County are striped on at least the anterior 30 percent of the body. The venter is black with a central row of large cream to yellowish spots. As in the case of the dorsal striping, this ventral pattern is best developed anteriorly and tends to break down posteriorly. The red pigmentation characteristic of mangrove water snakes is conspicuously lacking in Atlantic salt marsh snakes from the vicinity of Edgewater, Volusia County, and northward.
Hebrard (1979) reported coloration for 23 specimens from the southern Indian River Lagoon, near the Volusia-Brevard county line. Of these, 7 (30 percent) exhibited orange or reddish pigmentation either dorsally or ventrally. It is unclear at this time (1993) whether the reddish pigmentation reported by Hebrard should be interpreted as indicating intergradation with the mangrove water snake. The series of 25 specimens for which Hebrard provided pattern descriptions had dorsal stripes on 0 to 100 percent of the body; only 8 (32 percent) had dorsal stripes on more than 30 percent of the body, but 3 (12 percent) reportedly had dorsal stripes on 100 percent of the body. (In terms of pattern formation, the vertebral stripe is actually the lighter background color which is visible between the two dark, dorsolateral stripes.)
Distribution
The species to which the Atlantic salt marsh snake belongs, N. clarkii, is found in a narrow coastal strip from southern Texas, east along the Gulf coast, around the Florida peninsula, and up the east coast of Florida at least as far as Volusia County's Halifax River.
Both Cope’s (1895) type series and the specimens used by Carr and Goin (1942) to resurrect N. c. taeniata came from the brackish coastal marshes of Volusia County, Florida. There is some uncertainty about the precise locality from which Cope’s specimens came, but Carr and Goin restricted the type locality to the vicinity of National Gardens, which lies near the north end of the Halifax River. Salt marsh snakes have not been documented to the north in southern Flagler County. The Carr and Goin series was collected on the barrier island at New Smyrna Beach. Recent records for populations identifiable as Atlantic salt marsh snakes are available from (1) the barrier island a short distance north of Ponce Inlet, (2) the mainland shoreline east ofthe New Smyrna Beach airport, (3) two localities on the barrier island at New Smyrna Beach (Florida Game and Fresh Water Fish Commission (FGFWFC) records), (4) an island in the Indian River east of Edgewater (G. Goode, East Volusia Co. Mosquito Control, pers. comm.) and (5) a single specimen identified as N. c. taeniata was captured just south of the Flagler County line (G. Goode pers. comm.). It is not known if a viable population exists in this area or to the north in Flagler County but if so, these Atlantic salt marsh snakes are now isolated from populations in the northern Indian River Lagoon by the Ormond Beach-Daytona metropolitan area.
A problem attendant to the listing of any subspecies that is distributionally continuous and intergradient with another subspecies is the difficulty of defining the limit(s) of the listed form’s distribution in the area where it contacts the related, unlisted subspecies. To the south, the Atlantic salt marsh snake intergrades with the mangrove water snake along the central Atlantic coast of Florida. As noted above~ both the description and the resurrection of the subspecies were based on specimens from Volusia County, although Carr and Goin (1942) considered a single specimen from Indian River County also to be N. c. taeniata. They also mentioned a specimen of salt marsh snake from Melbourne, Brevard County, but did not indicate whether they considered that specimen to be N. c. taeniata. Wright and Wright (1957) considered N. c. taeniata to extend only as far south as the lower end of Mosquito Lagoon, in northern Brevard County, and Neill (1958) indicated that N. c. taeniata intergraded with the mangrove water snake on Merritt Island. In the final listing of the Atlantic salt marsh snake (FR 42:60743-60745), the Service indicated that “The Atlantic salt marsh snake is known only from coastal areas of Brevard, Volusia, and Indian River counties.” However, Hebrard and Lee (1981) examined a large series of salt marsh snakes from southern Mosquito Lagoon near the Volusia-Brevard county line and reported that they resembled Nerodia fasciata compressicauda quite closely.” Hebrard and Lee further noted that their specimens differed markedly in coloration and pattern from specimens of N. c. taeniata from further north in Volusia County. It is also worth noting that the snakes examined by Hebrard and Lee were collected in mangroves (species not indicated), whereas only about 10 miles farther north, where populations of typical Atlantic salt marsh snakes are found, the habitat consists primarily of glasswort (Salicornia spp.) flats and salt grass (Distichlis spicata)-bordered tidal creeks with only scattered black mangroves (Avicennnia germinans). The zone of intergradation appears to coincide with the increasing dominance of mangrove swamps, eventually as mangrove swamps become predominant so does N. c. compressicauda. Kochman (1992) concluded that “salt marsh snakes from farther south in Brevard and Indian River counties, although occasionally striped, appear to comprise a zone of intergradation with N. c. compressicauda.”
Until a survey and taxonomic assessment have been conducted, it will not be possible to determine the southern distributional limit of the Atlantic salt marsh snake. Nonetheless, it appears that the subspecies may be restricted to the brackish, coastal marshes of Volusia County, from the Halifax River south to the northern portions of the Indian River Lagoon.
Habitat
Atlantic salt marsh snakes are restricted to brackish, tidal marshes. They most often have been found in association with saltwort flats and salt grass-bordered tidal creeks. It is not known if they occur in the adjacent black needlerush (Juncus roemerianus) habitat. Atlantic salt marsh snake use of marsh habitats may be limited by water level; with extreme fluctuations making the marsh too hydric or xeric (G. Goode pers. comm.). When inactive or pursued, they frequently retreat into one of the numerous fiddler crab (Uca pugilator) burrows that riddle the edge of the marsh and the banks of the tidal creeks (Carr and Goin 1942, Kochman 1992, P. Moler pers. obs.).
Life History/Ecology
Although the Atlantic salt marsh snake is most easily observed at night, it may be active at any time of day. Its activity is influenced by tidal cycles, which strongly influence the availability of food (Neill 1958). Although Carr and Goin (1942) indicated that all of their specimens were collected “just as the tide was beginning to overflow the flats,” Kochman (1992) indicated that it was observed most often “during low tidal stages, when it apparently feeds on small fishes that become trapped in the shallow water.” It feeds primarily on small fish, but it readily takes frogs when available.
This species is ovoviviparous. Captive individuals have given birth to 3 to 9 young from August to October (Kochman 1992). Fecundity is low relative to the adjacent freshwater species, N. fasciata, which may give birth to 50 or more young.
Most snakes adapted to life in salt water (families Hydrophiidae, Achrocordidae, and Homalopsidae) possess salt glands, through which they excrete excess salts (Dunson 1975). The salt marsh snakes apparently lack salt glands (Schmidt-Nielsen and Fange 1958), but they nonetheless exhibit very low dehydration rates in seawater (Pettus 1963, Dunson 1978, 1980). Salt marsh snakes are apparently able to survive in seawater through their reduced rates of cutaneous water and salt exchange and their refusal to drink seawater even when they become dehydrated. By contrast, when held in seawater, their freshwater congeners quickly become dehydrated, which prompts them to drink. This merely exacerbates their dehydration and leads to death (Pettus 1963). Salt marsh snakes readily drink fresh water when it becomes available from rain or dew (Kochman 1992).
Threats
The Atlantic salt marsh snake was listed on the basis of two primary concerns, intensive drainage and development in coastal salt marshes resulting in loss of habitat and the accompanying disruption of reproductive isolating mechanisms, leading to hybridization with the Florida banded water snake and potential swamping ofthe Atlantic salt marsh snake gene pool by the much larger Florida banded water snake gene pool.
At the time of its listing, the Atlantic salt marsh snake was thought to include salt marsh snakes as far south as Indian River County (U.S. Fish Wildlife Service 1977). As suggested above, it may actually be much more restricted, occurring only in the brackish, coastal marshes of Volusia County. If so, then given its highly restricted distribution, the Atlantic salt marsh snake’s vulnerability to habitat destruction and modification is even greater than previously realized.
It is well known that salt marsh snakes occasionally hybridize with the closely related freshwater species, Nerodia fasciata, especially in areas of habitat disturbance (Kochman 1977, Dunson 1979, Lawson et al. 1991). Lawson ~ ~j. (1991) demonstrated that, despite the reproductive compatibility of the two forms, there appears to be little or no genetic introgression between them in areas of undisturbed habitat. The extent of genetic introgression associated with the local breakdown of reproductive isolation between the two species has not yet been examined.
Rising sea levels are not an immediate threat but in the long termmay reduce the amount of habitat available to the Atlantic salt marsh snake. As sea levels rise, salinity in the estuaries will also rise correspondingly and possibly change the vegetation ofthe marsh, eventually flooding the area and making it inhospitable for the snake.
Conservation Measures
Conservation measures have consisted of limited survey work, genetic comparison with other salt marsh snakes and southern banded water snakes, use ofthe provisions under Section 7 of the Act, Section 404 of the Clean Water Act (CWA), and the Fish and Wildlife Coordination Act (FWCA), and proposals for creation of habitat to mitigate for areas impacted by permitted dredge-and-fill activities.
Sporadic surveys conducted from 1978 to 1988 by personnel of the FGFWFC and the Service confirmed the continued presence of the Atlantic salt marsh snake at several localities in Volusia County, Florida. Personnel of the East Volusia County Mosquito Control District are currently conducting surveys for Atlantic salt marsh snakes associated with mosquito control impoundments on islands in the northern portions of the Indian River Lagoon (G. Goode pers. comm.). A survey was conducted on Merritt Island National Wildlife Refuge in the late 1970’s, and a large population of salt marsh snakes was identified in the vicinity of the Volusia-Brevard county line, but this population seemed to show signs of intergradation with the mangrove water snake (Hebrard and Lee 1981).
Localities in the vicinity of New Smyrna Beach were sampled by FGFWFC for genetic studies (Lawson et M. 1991). Electrophoretic analyses indicated that the salt marsh snakes are closely related to but specifically distinct from the southern banded water snake (Nerodia fasciata), and that the three subspecies of the salt marsh snake are electrophoretically indistinguishable from each other (Lawson et al. 1991). Tissues were saved for possible comparison of mitochondrial DNA variation in the salt marsh snakes, but that work has not yet been performed.
The Atlantic salt marsh snake is protected as a threatened species under the Endangered Species Act of 1973, as amended (16 U.S.C. 1531 ~4seq.). The Act places an affirmative mandate on Federal agencies to carry out programs for the conservation of federally listed endangered and threatened species. Further, the Act requires all Federal agencies to ensure that their actions are not likely to jeopardize the continued existence of any federally listed endangered or threatened species. Federal agency actions that can directly or indirectly affect endangered or threatened species include any activity that is authorized, funded, or carried out by such agency. Compliance with theses standards is ensured under Section 7 of the Act because agencies must consult with the Service or National Marine Fisheries Service on actions that may affect listed species or critical habitat.
In addition to Section 7 consultations, protection and conservation of salt marsh habitat is provided by CWA and FWCA. The Service and U.S. Army Corps of Engineers review proposed dredge-and-fill activities and construction projects in waters of the United States where projects may affect the Atlantic salt marsh snake or its habitat. During a 10-year period (1983-1992) a minimum of 36 various projects were permitted in Volusia County’s salt marsh habitat. These projects included dredge-and-fill, shoreline protection projects, construction of piers and marinas, mosquito ditching, and water control structures. However, only 32 acres of salt marsh were destroyed by these projects, most (29.44 acres, 18 projects) before 1988. Loss of salt marsh habitat appears to have slowed since 1988 (2.56 acres, 18 projects) indicating improved protection. If the Atlantic salt marsh snake is limited to Volusia County, any project destroying salt marsh habitat may be detrimental to the species.